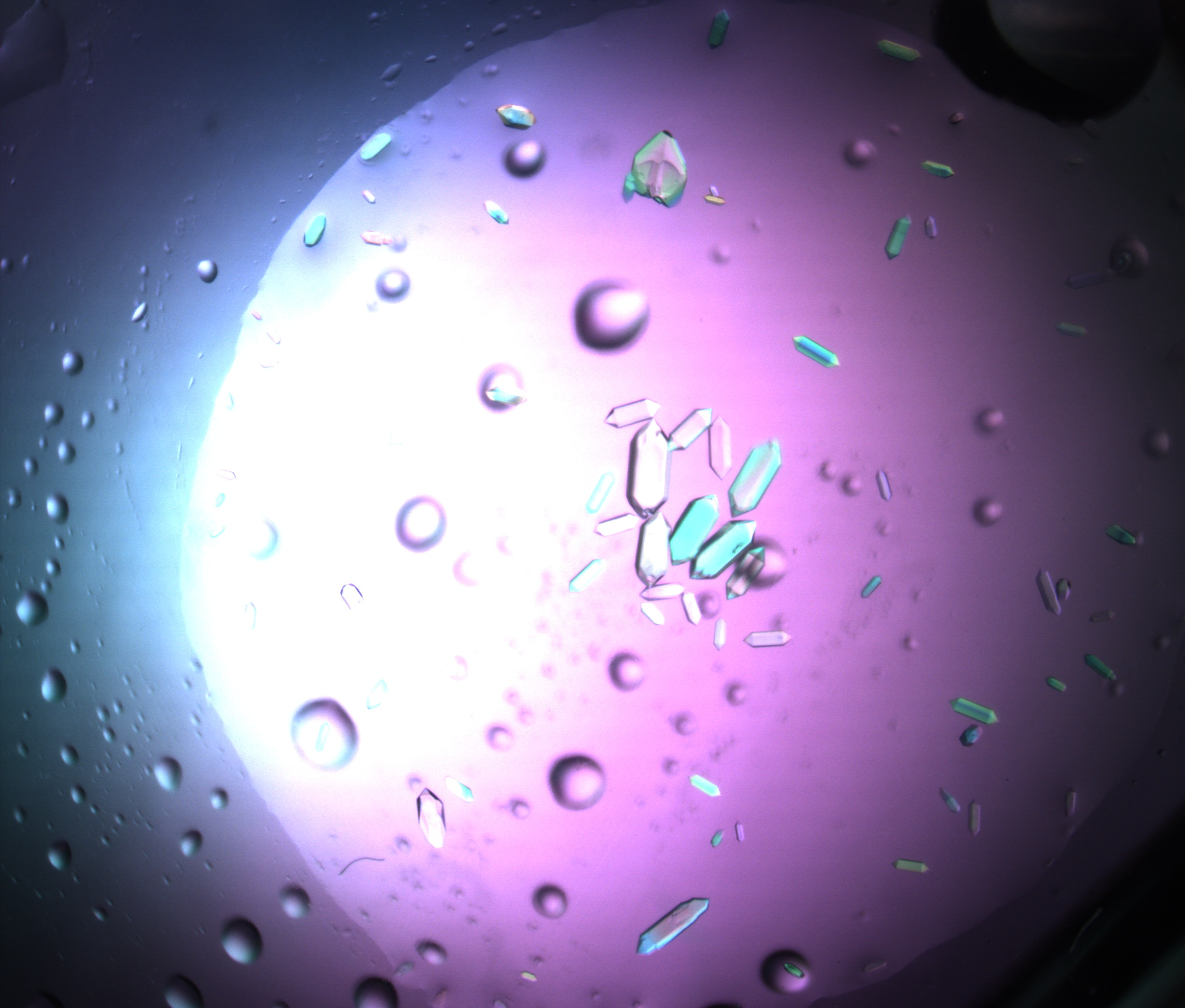
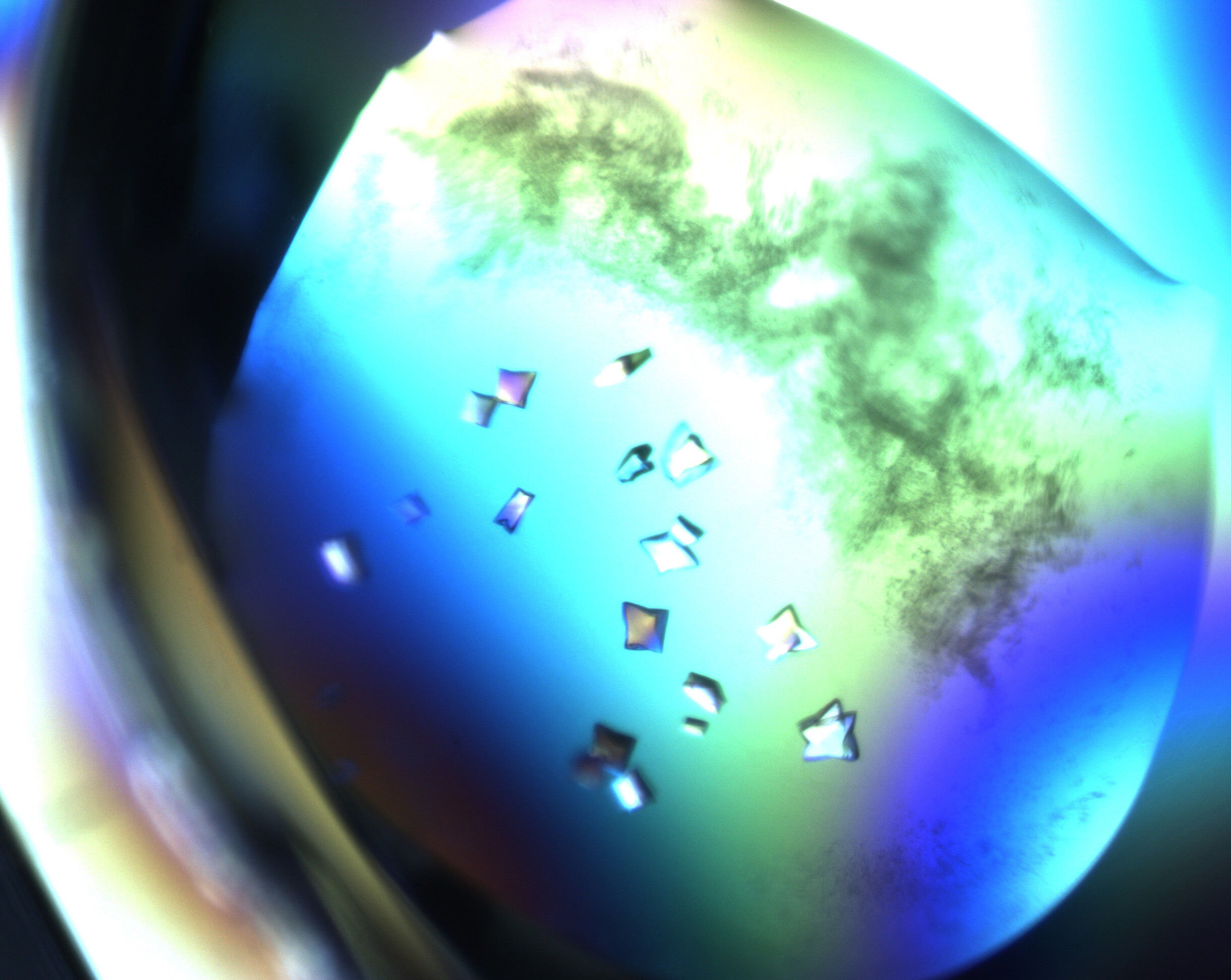
The road to science is paved with good samples. For NMX, the Neutron Macromolecular Crystallography instrument at ESS, this means preparing protein crystals well in advance of neutrons being produced. With the sample preparation area now operational, crystal growth is underway. This is a time-critical step to ensure that NMX will be ready for its first science.
The sample preparation lab has been fitted with benches, incubators, cupboards, microscopes, and a liquid handling robot for protein crystal growth. These crystals, produced from purified proteins, must be nurtured carefully over many months. It can take between six to 18 months for a single crystal to be ready to be mounted onto the sample stage for experiments. Beginning this work now, with in-house teams and collaborators at partner institutes, ensures that NMX will have the right samples in time for commissioning tests, before welcoming external researchers in 2028.
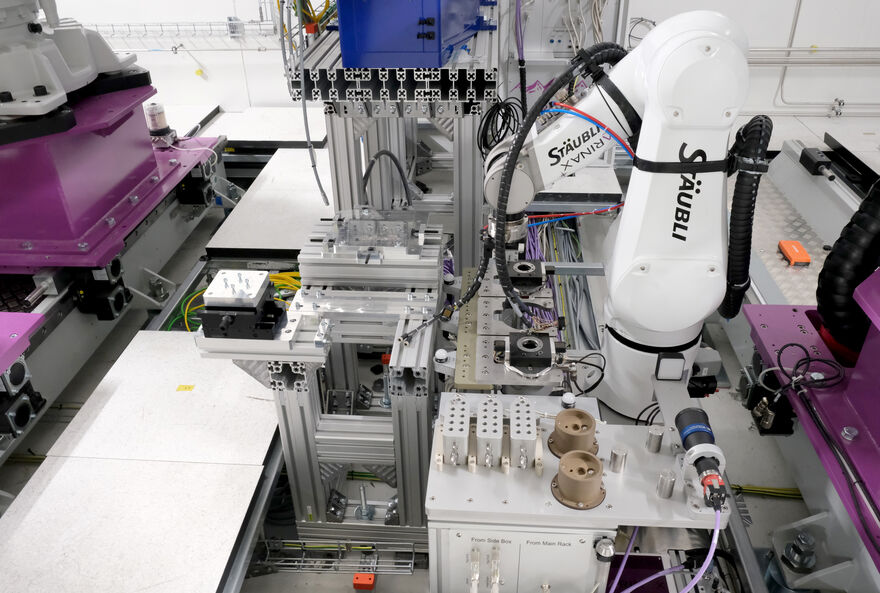
While crystals are growing in the lab, installation continues in and around the NMX instrument itself. Inside the cave, robotic systems for handling samples and positioning neutron detectors are now in place and will soon be energised to begin motion testing. These include a precision robotic arm capable of transferring crystals from a sample holder to the beam position with micrometre accuracy.
In the building adjacent to the cave, the ground floor houses the new sample preparation area where crystals are being grown and mounted for experiments. On the upper floor is the local control hutch, which will be equipped with monitoring stations where researchers will control the instrument, collect and assess diffraction data in real time.
What does NMX do?
NMX uses neutrons to study the 3D atomic structure of large biological molecules such as proteins and DNA. Its unique strength lies in the ability to locate hydrogen atoms, which are often invisible to other techniques like X-ray crystallography. This enables detailed mapping of hydrogen bonds – crucial interactions that play a central role in protein folding, structure, and function. These details are vital for understanding how enzymes function and how drugs bind to clinical target proteins. Through detailed analysis of the neutron crystal structures, scientists can reconstruct the complete atomic layout of these molecules to gain new insights into biology and drug design.
Applications will range from drug design, industrial enzymes, food science, and fundamental mechanistic studies of how enzymes work.
Everyday examples of what NMX can study:
Drug design and Medicine: Understanding how drugs bind - and potentially inhibit - a clinically relevant protein. This can guide the design of improved drugs and support discovery of new treatments. Pinpointing exactly where hydrogens are can make the difference between a good drug and a great one.
Fundamental Biology: Seeing the precise structure of enzymes and proteins involved in essential processes that support living cells. This is useful to understand how cells behave, from ageing to metabolism, and how mutations can drive certain diseases such as cancer.
Environment & Food Science: Studying plant proteins or enzymes used in industrial processes can enable a deeper understanding and ultimately drive optimisation of bio-catalysts with enhanced functional properties.
NMX was developed in collaboration with several in-kind partners across Europe. Its design builds on expertise in existing neutron diffraction platforms.
Read more about NMX here: https://ess.eu/instruments/nmx